Band-bowing effects in lead-free double Cs2AgBixSb1−xCl6 perovskites and their anion-exchanged derivatives†
Abstract
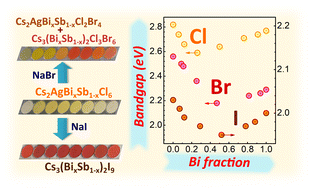
Green synthesis of lead-free Cs2AgBixSb1−xCl6 solid-solution double perovskites and their conversion into bromide and iodide derivatives is reported. Interaction of the single-phase Cs2AgBixSb1−xCl6 double perovskites with sodium iodide in 2-propanol/glycerol yields Cs3Bi2xSb2(1−x)I9 derivatives with varied Bi/Sb ratio, while the similar anion exchange with sodium bromide results in mixtures of Cs3Bi2xSb2(1−x)Cl3Br6 and Cs2AgBixSb1−xCl2Br4. A band-bowing effect is revealed for the starting chloride perovskites and the anion-exchange products, with the indirect band gap of intermediate Bi/Sb mixed compounds being significantly lower than the band gaps of corresponding Bi- and Sb-pure products. Solid-state nuclear magnetic resonance spectroscopy uncovered that the band-bowing originates from the lattice disorder of mixed Bi/Sb halide compounds, thus highlighting a new approach for the bandgap design of halide perovskites.


 Please wait while we load your content...
Please wait while we load your content...