Macrocyclic luminophores under confinement in a polymeric matrix – induction of large-Stokes shift by inter-unit proton transfer†
Abstract
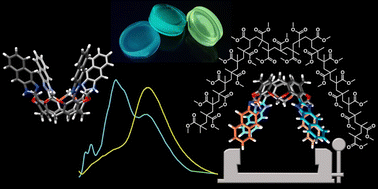
We demonstrate that semi-rigid macrocyclic compounds, tetra(naphthimidazole)resorcin[4]arenes, consisting of four naphthimidazole-resorcinol units, exhibit large bathochromic shifts of emission upon embedding in the polymeric matrix. The mechanism of generation of the large Stokes shift involves inter-unit proton transfer that leads to charge-separated emissive species. This process requires proximity of the units and intramolecular stabilization of the charge-separated tautomers and therefore is unique for the macrocyclic geometry. The mechanism of tautomerization was verified experimentally and supported by theoretical calculations of the absorption and emission properties obtained at the CC2/aug-cc-pVDZ theory level which proved that intramolecular inter-unit proton transfer leads to the formation of emissive cationic and anionic groups within the macrocycle. The process is observed in the solution, but it is considerably enhanced by a constrictive environment, allowing the synthesis of luminophore-doped PMMA materials having features that are prerequisites for applications as luminescent solar concentrators – they absorb in the UV-A region and emit in the visible range resulting in visibly transparent fluorescent polymeric materials exhibiting large Stokes shifts (∼180 nm) with minimized re-absorption losses.
- This article is part of the themed collection: #MyFirstJMCC


 Please wait while we load your content...
Please wait while we load your content...