New shades of photochromism – yellow sodalites for the detection of blue light†
Abstract
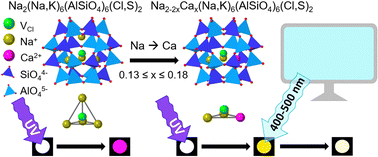
The absorption energy of the F-center in photochromic sodalite can be lowered by expanding the unit cell with larger ions, changing the color from pink to blue. Blue-shifting this absorption to produce other colors is not so straightforward. In this work, sodalites of the formula Na2−2xCax(Na,K)6(AlSiO4)6(Cl,S)2 displaying white-to-yellow photochromism have been prepared and thoroughly characterized. Combining spectroscopic experiments and quantum chemical calculations, the formation of a Na2Ca entity inside the sodalite cage surrounding the trapped electron responsible for the yellow color is postulated. Optimal yellow photochromism occurs for 0.13 ≤ x ≤ 0.18, while at x ≥ 0.27 the formation of the by-product davyne begins to affect the structure and optical properties. Finally, the sensitivity of these materials to blue light is shown to make them well-suited as sensors for blue light over-exposure e.g. from computer screens or smart phones.


 Please wait while we load your content...
Please wait while we load your content...