Superior gene transfection efficiency in triple negative breast cancer by RAFT-mediated amino acid-based cationic diblock copolymers†
Abstract
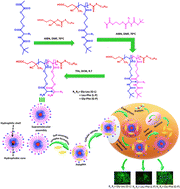
To date, the synthesis of efficient and safe gene carriers with low toxicity and appreciable gene transfection efficiency has been the major hurdle associated with non-viral gene carriers. Herein, we synthesized three amino acid-based diblock copolymers comprising glycine–leucine, leucine–phenyl alanine and glycine–phenyl alanine group containing blocks. The synthesis of all the diblock copolymers was confirmed by FTIR, 1H NMR, DLS and GPC techniques. All the polymers showed a high positive zeta potential value that varies from 45 ± 1 mV to 56 ± 1 mV, and the hydrodynamic size of the polymers varies from 250 ± 8 to 303 ± 14 nm. The three polymers showed negligible cytotoxicity compared with PEI (25 kDa) for MDA-MB-231 and NKE cells. Among all other polymers, P(HGN)n-b-P(HPN)m exhibited the highest biocompatibility with ∼70% cell viability at a concentration of 200 μg mL−1. Hemolysis data revealed that among all three polymers, P(HGN)n-b-P(HPN)m exhibited the highest blood compatibility, while up to a high concentration of 200 μg mL−1, it showed a very negligible amount (∼18%) of hemolysis. Most importantly, excellent gene complexation capability and good protection of pDNA against enzymatic degradation were observed with all three diblock copolymers. Interestingly, P(HGN)n-b-P(HPN)m/pDNA complex showed the smallest particle size (∼15 nm) and highest positive zeta potential as observed from TEM micrographs and DLS analysis, which probably results significantly higher level of cellular uptake and hence the highest transfection efficiency (∼85%) against MDA-MB-231 cells. Therefore, the diblock copolymer P(HGN)n-b-P(HPN)m with superior gene transfection efficiency in triple negative breast cancer may be an efficient non-viral vector for successful TNBC therapy in the future.


 Please wait while we load your content...
Please wait while we load your content...