Mechanistic insights and importance of hydrophobicity in cationic polymers for cancer therapy†
Abstract
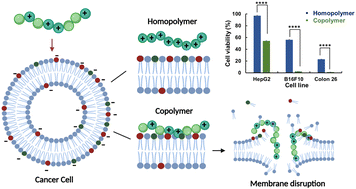
Development of molecules that can be effectively used for killing cancer cells remains a research topic of interest in drug discovery. However, various limitations of small molecules and nanotechnology-based drug-delivery systems hinder the development of chemotherapeutics. To resolve this issue, this study describes the potential application of polymeric molecules as anticancer drug candidates. We describe the design and synthesis of novel anticancer polymers containing hydrophobic groups. We established the fact that the cationic homopolymer (PAMPTMA) does not show any anticancer activity on its own; however, the insertion of hydrophobic moieties in copolymers (PAMPTMA-r-BuMA, PAMPTMA-r-HexMA, and PAMPTMA-r-OctMA) enhances their anticancer activity with a very low IC50 value (60 μg mL−1 for HepG2 cells). Mechanistic investigations were carried out using LDH leakage assay, cellular uptake, DOSY NMR and molecular dynamics to study the interaction between the polymer and the cell membrane as well as the role of hydrophobicity in enhancing this interaction. The results demonstrated that polymers are attracted by the anionic cancer cell membrane, which then leads to the insertion of hydrophobic groups inside the cell membrane, causing its disruption and ultimate lysis of the cell. This study demonstrates a novel and better approach for the rational design and discovery of new polymeric anticancer agents with improved efficacy.


 Please wait while we load your content...
Please wait while we load your content...