Identifying hexagonal 2D planar electrocatalysts with strong OCHO* binding for selective CO2 reduction†
Abstract
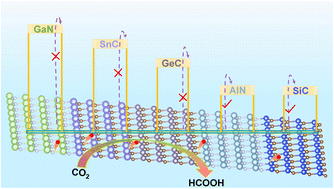
The efficient electrochemical conversion of carbon dioxide (CO2) into beneficial fuels and feedstocks underpins an economically profitable strategy to foster carbon neutrality and meet the burgeoning energy demand. However, finding alternative catalysts that can exhibit comparable catalytic activity to commercial precious metal catalysts remains a giant challenge for the CO2 reduction reaction (CO2RR). Two-dimensional (2D) materials with high specific surface area and thickness-tunability show great potential in the CO2RR. Herein, we perform a computational screening of 2D monolayer materials (AlN, GaN, InN, SiC, GeC, and SnC) via surface Pourbaix analyses, identifying five materials with available sites under operating conditions. We delved into the catalytic activity of these surfaces and found that they possess a strong affinity for bridge-adsorbed OCHO* intermediates owing to the two O*-surface bonds possessing markedly negative ICOHP values. In contrast, these surfaces generally bound *COOH and H* weakly. This discrepancy in adsorption strengths is highly efficacious in curtailing the undesired hydrogen evolution reaction whilst predisposing the CO2RR to follow the OCHO* pathway more readily. AlN and SiC emerge as promising catalysts for formic acid (HCOOH) production, boasting low reaction free energies of the rate-determining step (ΔGMAX: 0.48 and 0.47 eV, respectively). Moreover, CH4 formation is also favored on monolayer SiC with a ΔGMAX of 0.41 eV, and the performance of monolayer SiC is higher than that of multilayer SiC. This study provides new insights into the theoretical foundation to effectively design and screen highly active and product-selective 2D catalysts.
- This article is part of the themed collection: 2023 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...