Continuous wet chemical synthesis of Mo(C,N,O)x as anode materials for Li-ion batteries†
Abstract
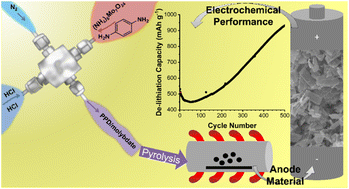
Molybdenum carbides, oxides, and mixed anionic carbide–nitride–oxides Mo(C,N,O)x are potential anode materials for lithium-ion batteries. Here we present the preparation of hybrid inorganic–organic precursors by a precipitation reaction of ammonium heptamolybdate ((NH4)6Mo7O24) with para-phenylenediamine in a continuous wet chemical process known as a microjet reactor. The mixing ratio of the two components has a crucial influence on the chemical composition of the obtained material. Pyrolysis of the precipitated precursor compounds preserved the size and morphology of the micro- to nanometer-sized starting materials. Changes in pyrolysis conditions such as temperature and time resulted in variations of the final compositions of the products, which consisted of mixtures of Mo(C,N,O)x, MoO2, Mo2C, Mo2N, and Mo. We optimized the reaction conditions to obtain carbide-rich phases. When evaluated as an anode material for application in lithium-ion battery half-cells, one of the optimized materials shows a remarkably high capacity of 933 mA h g−1 after 500 cycles. The maximum capacity is reached after an activation process caused by various conversion reactions with lithium.
- This article is part of the themed collection: #MyFirstJMCA


 Please wait while we load your content...
Please wait while we load your content...