Metal cation substitution can tune CO2, H2O and CH4 switching pressure in transiently porous coordination networks†
Abstract
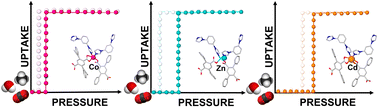
Compared to rigid physisorbents, switching coordination networks that reversibly transform between closed (non-porous) and open (porous) phases offer promise for gas/vapour storage and separation owing to their improved working capacity and desirable thermal management properties. We recently introduced a coordination network, X-dmp-1-Co, which exhibits switching enabled by transient porosity. The resulting “open” phases are generated at threshold pressures even though they are conventionally non-porous. Herein, we report that X-dmp-1-Co is the parent member of a family of transiently porous coordination networks [X-dmp-1-M] (M = Co, Zn and Cd) and that each exhibits transient porosity but switching events occur at different threshold pressures for CO2 (0.8, 2.1 and 15 mbar, for Co, Zn and Cd, respectively, at 195 K), H2O (10, 70 and 75% RH, for Co, Zn and Cd, respectively, at 300 K) and CH4 (<2, 10 and 25 bar, for Co, Zn and Cd, respectively, at 298 K). Insight into the phase changes is provided through in situ SCXRD and in situ PXRD. We attribute the tuning of gate-opening pressure to differences and changes in the metal coordination spheres and how they impact dpt ligand rotation. X-dmp-1-Zn and X-dmp-1-Cd join a small number of coordination networks (<10) that exhibit reversible switching for CH4 between 5 and 35 bar, a key requirement for adsorbed natural gas storage.


 Please wait while we load your content...
Please wait while we load your content...