A strong–weak binary solvation structure for unimpeded low-temperature ion transport in nanoporous energy storage materials†
Abstract
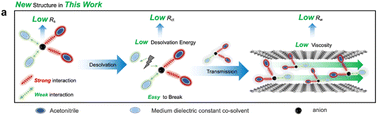
Proper balance between ionic conductivity and desolvation energy is critical for ion transport in nanoporous electrodes, which determines the tolerance of electrochemical energy storage devices to low temperatures. To achieve this balance, we propose a new concept of strong–weak binary solvation structure, where the ion's solvation structure comprises strong- and weak-interaction solvents simultaneously. The high-dielectric-constant solvent strongly solvates ions for promoting cation–anion dissociation and superior conductivity, while the medium-dielectric-constant solvent weakly interacts with ions, resulting in low desolvation energy for unimpeded ion transport in nanopores. This concept is verified by comprehensive studies of the solvation structures, thermodynamic properties, and ion transport dynamics in binary-solvent mixtures with different dielectric constants at different temperatures. We demonstrate that the strong–weak binary solvation structure strategy realizes a superior trade-off between the high ionic conductivity and low desolvation energy, leading to fast ion dynamics in nanopores at ultralow temperatures. As a proof-of-concept, we demonstrate that an activated carbon supercapacitor utilizing our binary solvation structure achieves remarkable capacitance retention (89% from 20 to −70 °C at 10 mV s−1), outstanding power density (6849.35 W kg−1 at 11.79 W h kg−1), and an ultralong cycling stability of 94.1% after 30 000 cycles at −70 °C.


 Please wait while we load your content...
Please wait while we load your content...