MnP4 electrode for Na-ion batteries: a complex and effective electrochemical mechanism†
Abstract
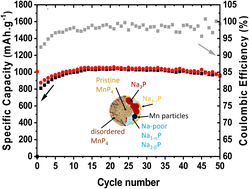
MnP4 has recently been identified as a possible negative electrode for Li-ion batteries. This study shows that this material can also perform as a negative electrode for Na-ion batteries (SIBs), demonstrating that a suitable electrode formulation allows an exceptional performance, i.e., a stable specific capacity of 1000 mA h g−1 after 50 cycles, much higher than that of hard-carbon-based anodes currently used in SIBs. The combination of operando X-ray diffraction, ex situ Mn K-edge X-ray absorption spectroscopy (XAS) and NMR spectroscopy reveals a complex conversion mechanism with the formation of numerous amorphous sodiated phosphide species. 31P and 13Na NMR were particularly effective in identifying these species, demonstrating that their formation is dependent on the preparation of the electrode, especially the amount of carbon additive. XAS analysis, on the other hand, proved the complete reversibility of the mechanism, including the reformation of MnP4 during the desodiation process.


 Please wait while we load your content...
Please wait while we load your content...