Deciphering ligand-controlled charge transfer from a metal–organic framework to cadmium sulfide for enhanced photocatalytic hydrogen evolution reaction†
Abstract
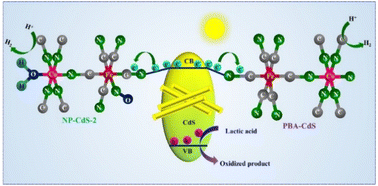
The suitable choice of a cocatalyst plays an important role in charge separation and transport and hence the hydrogen evolution activity of the photocatalyst. Herein, we have demonstrated the cocatalytic activities of two different metal–organic frameworks: CoFe–Prussian blue analog (CoFe–PBA) and CoFe–nitroprusside (CoFe–NP) loaded on CdS nanorods. The replacement of a –CN group of PBA with a strong π-acidic ligand, –NO, produces NP with a significant difference in the electronic structure. The introduction of –NO results in an increased positive charge density on the metal centers of CoFe–NP, making it more susceptible to charge carrier transport from CdS through strong back donation than CoFe–PBA. Under the best reaction conditions, NP-CdS-2 produces hydrogen at a rate of 1735 μmol g−1 h−1, significantly higher than those of bare CdS and PBA–CdS. Moreover, transient absorption spectroscopy has revealed a long-lived charge separated state in NP-CdS-2 to enhance the photocatalytic activity.
- This article is part of the themed collection: #MyFirstJMCA


 Please wait while we load your content...
Please wait while we load your content...