Intermediate-temperature proton conductivity of Li+/H+ ion-exchanged material (Li,H)3.5Zn0.25GeO4†
Abstract
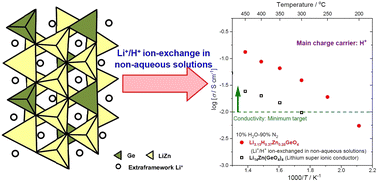
For decades, much effort has been devoted to developing proton conductors applicable to electrochemical devices at intermediate temperatures. However, promising materials that possess sufficient conductivity have not been realized yet. This study demonstrates the development of novel proton conductors that are operative at intermediate temperatures, especially 300–400 °C, through the simple ion-exchange method. The Li+/H+ ion-exchange process was conducted for Li14Zn(GeO4)4, one of the members of lithium super ionic conductors (LISICONs), in non-aqueous solutions. The chemical formula of the resultant sample was determined as Li3.13H0.37Zn0.25GeO4 from instrumental analyses. The electrical conductivity of this material was evaluated to be 87.0 mS cm−1, 39.0 mS cm−1, and 5.5 mS cm−1 at 400 °C, 300 °C, and 200 °C, respectively, in 10% H2O–90% N2. Furthermore, the main charge carrier in this electrolyte was identified as a proton from the H/D isotopic exchange study. These findings open up the possibility of realizing new electrochemical devices that are operative at 200–400 °C.


 Please wait while we load your content...
Please wait while we load your content...