Constant-potential molecular dynamics simulation and its application in rechargeable batteries
Abstract
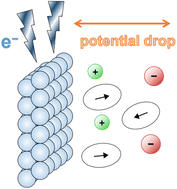
With the growing demand for energy storage technologies, current batteries have significant room for improvement. To find battery materials that offer high energy densities, fast rates, and long life spans, fundamental knowledge of battery interfaces is essential, as they largely determine the stability and rate performance of the battery. Molecular dynamics (MD) simulation is a powerful tool to study the atomistic phenomena occurring at battery interfaces, but the conventional uncharged MD methods fail to provide a realistic illustration of the Coulomb interactions between the electrode and electrolyte atoms. To fill this gap, several constant-potential MD methods have been developed over the last three decades aimed at truly representing the electrified electrochemical interface. These methods—both for classical MD and for ab initio MD—are discussed in this review, and their applications in rechargeable batteries are summarized. They can be used to elucidate interfacial phenomena including the structure of the electric double layer, the growth of the solid electrolyte interphase, and the occurrence of metallic dendrites. A battery's interfacial properties and interfacial reactions can also be probed using such methods. Finally, prospects for the modeling of battery interfaces are discussed, with the goal of making the MD model more physically accurate and computationally efficient.
- This article is part of the themed collections: Journal of Materials Chemistry A Emerging Investigators, Journal of Materials Chemistry A Recent Review Articles and #MyFirstJMCA


 Please wait while we load your content...
Please wait while we load your content...