Understanding surface chemical processes in perovskite oxide electrodes†
Abstract
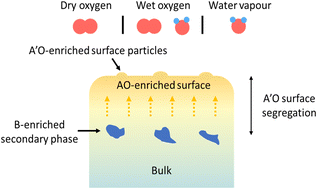
The effect of operating conditions on the surface composition and evolution of (La0.8Sr0.2)0.95Cr0.5Fe0.5O3−δ (LSCrF8255) as a model perovskite oxide was investigated. LSCrF8255 pellets were annealed under dry oxygen (pO2 = 200 mbar), wet oxygen (pO2 = 200 mbar, pH2O = 30 mbar), and water vapour (pO2 < 1 mbar, pH2O = 30 mbar) environments to reflect the applications of perovskite materials as electrodes for oxygen reduction/evolution and H2O electrolysis in electrochemical energy conversion devices such as solid oxide fuel/electrolysis cells (SOFCs/SOECs) and oxygen transport membranes (OTMs). A series of comprehensive surface characterization techniques were applied, including low energy ion scattering spectroscopy (LEIS), X-ray photoelectron spectroscopy (XPS), secondary ion mass spectrometry (SIMS), scanning electron microscopy (SEM), scanning transmission electron microscopy (STEM), and energy-dispersive X-ray spectroscopy (EDX). Our comprehensive study showed that after annealing at 900 °C for 27 hours, a severe level of Sr surface segregation occurred on the sample annealed in both dry oxygen and water vapour but in different manners, whereas on the sample annealed in wet oxygen, Sr segregation was likely suppressed. In addition, the Sr segregation behaviour can be correlated to other mass transport phenomena, such as Cr evaporation and redeposition and Si deposition, as well as to crystal orientation and defects such as grain boundaries and dislocations. Apart from the Sr-enriched surface precipitates, phase separation was consistently observed on the samples annealed in all three conditions. The secondary phase was found to be B-site cation enriched (significantly Fe enriched, relatively Cr enriched) and A-site cation (La and Sr) deficient. Moreover, in contrast to the Sr enriched surface, a La enriched surface was observed on samples annealed in dry oxygen at 600 and 700 °C, which was found to be potentially caused by the Sr and Cr surface evaporation processes.


 Please wait while we load your content...
Please wait while we load your content...