New strategies to improve two-electron oxygen reduction reaction selectivity of polypyrrole-based catalysts†
Abstract
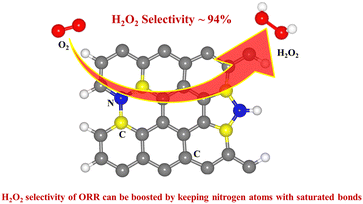
Direct electrochemical synthesis via the two-electron oxygen reduction reaction (ORR) is considered an environmentally friendly means to produce hydrogen peroxide (H2O2). Metal-free N-doped carbon materials are reported to display good electrocatalytic performance for H2O2 generation. Herein, we found that selectivity toward H2O2 production in the ORR can be further enhanced once the N atoms are saturated bonds in polypyrrole. Saturation can either be achieved by stabilizing the N–H bonds via hydrogen-bonding interactions during preparation or transforming the deprotonated nitrogen species into C–N bonds by calcining polypyrrole at appropriate temperatures. Eventually, our synthesized catalyst NPC-400 exhibits a 94% H2O2 selectivity and impressive stability. The mechanisms are elucidated by using control experiments and density-functional theory calculations. The α carbon atoms of pyrrolic-N are the active sites for selective H2O2 production. However, once the N atoms are not saturated, the OOH* intermediate species tend to bond with the N atoms, leading to relatively poor reaction selectivities. Our work provides new insight for designing high selectivity catalysts for H2O2 generation in the oxygen reduction reaction.


 Please wait while we load your content...
Please wait while we load your content...