Low-temperature sintering characteristics and electrical properties of Ca- and Bi-doped Li7La3Zr2O12 electrolyte containing Li3BO3 additive†
Abstract
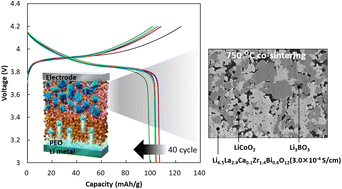
Li7La3Zr2O12 (LLZ) is one of the most promising Li-ion solid electrolytes for all-solid-state Li-ion batteries. To achieve low interfacial resistance between LLZ and the active material, low-temperature sintering is required. Toward this, we co-doped Ca and Bi into LLZ and used Li3BO3 as an additive to examine these species as sintering aids. We successfully demonstrated that Li6.5La2.9Ca0.1Zr1.4Bi0.6O12 containing Li3BO3 can be sintered even at 750 °C with a relative density of 89% and conductivity of 3 × 10−4 S cm−1 at 25 °C. Thermomechanical and microstructural analyses revealed that the starting temperature of the viscous flow, which was initiated by the formation of a liquid Li3BO3 phase, was reduced owing to the reaction between Ca2+ and Li3BO3. Additionally, the Li–Ca–Bi–O liquid phase formed between the LLZ particles during sintering improved the sinterability, which consequently promoted the necking between particles and lowered the grain boundary resistance. Additionally, we demonstrated that the developed LLZ was applicable as an electrolyte for all-solid-state lithium-ion batteries with high cycling stability. Thus, Li6.5La2.9Ca0.1Zr1.4Bi0.6O12 containing Li3BO3 is a promising candidate as an electrolyte for the cathode layer of all-solid-state lithium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...