Membrane permeabilization can be crucially biased by a fusogenic lipid composition – leaky fusion caused by antimicrobial peptides in model membranes†
Abstract
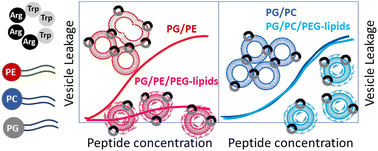
Induced membrane permeabilization or leakage is often taken as an indication for activity of membrane-active molecules, such as antimicrobial peptides (AMPs). The exact leakage mechanism is often unknown, but important, because certain mechanisms might actually contribute to microbial killing, while others are unselective, or potentially irrelevant in an in vivo situation. Using an antimicrobial example peptide (cR3W3), we illustrate one of the potentially misleading leakage mechanisms: leaky fusion, where leakage is coupled to membrane fusion. Like many others, we examine peptide-induced leakage in model vesicles consisting of binary mixtures of anionic and zwitterionic phospholipids. In fact, phosphatidylglycerol and phosphatidylethanolamine (PG/PE) are supposed to reflect bacterial membranes, but exhibit a high propensity for vesicle aggregation and fusion. We describe the implications of this vesicle fusion and aggregation for the reliability of model studies. The ambiguous role of the relatively fusogenic PE-lipids becomes clear as leakage decreases significantly when aggregation and fusion are prevented by sterical shielding. Furthermore, the mechanism of leakage changes if PE is exchanged for phosphatidylcholine (PC). We thus point out that the lipid composition of model membranes can be biased towards leaky fusion. This can lead to discrepancies between model studies and activity in true microbes, because leaky fusion is likely prevented by bacterial peptidoglycan layers. In conclusion, choosing the model membrane might implicate the type of effect (here leakage mechanism) that is observed. In the worst case, as with leaky fusion of PG/PE vesicles, this is not directly relevant for the intended antimicrobial application.


 Please wait while we load your content...
Please wait while we load your content...