A theoretical model of efficient phagocytosis driven by curved membrane proteins and active cytoskeleton forces†
Abstract
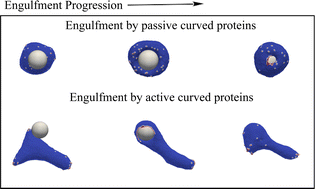
Phagocytosis is the process of engulfment and internalization of comparatively large particles by cells, and plays a central role in the functioning of our immune system. We study the process of phagocytosis by considering a simplified coarse grained model of a three-dimensional vesicle, having a uniform adhesion interaction with a rigid particle, and containing curved membrane-bound protein complexes or curved membrane nano-domains, which in turn recruit active cytoskeletal forces. Complete engulfment is achieved when the bending energy cost of the vesicle is balanced by the gain in the adhesion energy. The presence of curved (convex) proteins reduces the bending energy cost by self-organizing with a higher density at the highly curved leading edge of the engulfing membrane, which forms the circular rim of the phagocytic cup that wraps around the particle. This allows the engulfment to occur at much smaller adhesion strength. When the curved membrane-bound protein complexes locally recruit actin polymerization machinery, which leads to outward forces being exerted on the membrane, we found that engulfment is achieved more quickly and at a lower protein density. We consider spherical and non-spherical particles and found that non-spherical particles are more difficult to engulf in comparison to the spherical particles of the same surface area. For non-spherical particles, the engulfment time crucially depends on the initial orientation of the particles with respect to the vesicle. Our model offers a mechanism for the spontaneous self-organization of the actin cytoskeleton at the phagocytic cup, in good agreement with recent high-resolution experimental observations.


 Please wait while we load your content...
Please wait while we load your content...