Low-temperature thermal oxidation of biomass jet fuel pinane†
Abstract
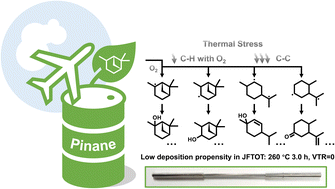
Pinane is a product derived from turpentine, a renewable resource that is highly valued. Due to its unique multicyclic ring structure, pinane has a relatively high energy density of 35.6 MJ L−1, making it an excellent potential alternative to jet fuel. In this work, the low-temperature oxidation of pinane is comprehensively studied concerning the oxidative stability requirement of jet fuels. The apparent activation energy Ea and pre-exponential factor A of pinane oxidation are acquired according to the Kissinger method using a P-DSC apparatus as 97.6 ± 2.7 kJ mol−1 and 5.3 ± 3.7 × 1010 min−1, respectively, rendering it a relatively more oxygen-susceptible hydrocarbon compared to non-strained hydrocarbons. DFT calculations and experimental investigations have shown that the low-temperature oxidation of pinane begins with the hydrogen atom transfer (HAT) reaction and the rupture of the strained ring. Interestingly, although pinane can be easily oxidized, it shows very low deposition propensity in the JFTOT test, qualifying it as a potential jet fuel.


 Please wait while we load your content...
Please wait while we load your content...