Efficient syngas production with controllable CO : H2 ratios based on aqueous electrocatalytic CO2 reduction over mesoporous TiO2 films modified with a cobalt porphyrin molecular catalyst†
Abstract
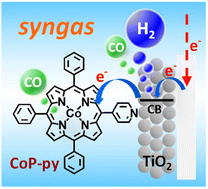
There is an industrial demand to develop a facile method to produce syngas having a specific CO : H2 ratio for the sake of synthesizing various value-added chemicals. Especially, it remains a challenge to invent truly earth-abundant and environmentally friendly routes to overcome this issue. Here we report on a technique enabling electrochemical syngas production from a neutral aqueous CO2-saturated NaHCO3 solution based on the combined use of a pristine and a catalyst-anchored mesoporous TiO2 electrode. Our study achieves a relatively high CO ratio (CO : H2 = 53 : 47) using a pyridyl-modified cobalt porphyrin CO2 reduction catalyst anchored over TiO2 films, which can largely suppress the H2 evolution based on its high selectivity in CO production even in fully aqueous media. We demonstrate the excellent tunability of the CO : H2 ratio in the range of 1 : 1 to 1 : 20 by simply tuning the electrolysis potential together with the associated conditions.


 Please wait while we load your content...
Please wait while we load your content...