para-Selective dearomatization of phenols by I(i)/I(iii) catalysis-based fluorination†
Abstract
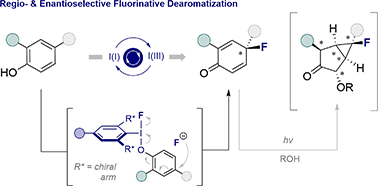
The regio- and enantio-selective dearomatization of phenols has been achieved by I(I)/I(III) catalysis enabled fluorination. The process is highly para-selective, guiding the fluoride nucleophile to the distal C4 position of the substrate to generate fluorinated cyclohexadienones in an operationally simple manner. Extensive optimization has revealed key parameters that orchestrate enantioselectivity in this historically challenging transformation. A range of diversely substituted substrates are disclosed (20 examples, up to 92 : 8 e.r.) and the reaction displays efficiency that is competitive with the current state of the art in hydroxylation chemistry: this provides a preparative platform to enable OH to F bioisosterism to be explored. Finally, the utility of the products in accessing densely functionalized cyclic scaffolds with five contiguous stereocenters is disclosed together with crystallographic analyses to unveil fluorine-carbonyl non-covalent interactions.


 Please wait while we load your content...
Please wait while we load your content...