Cobalt-catalyzed decarboxylative difluoroalkylation of nitrophenylacetic acid salts†
Abstract
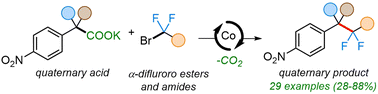
The selective installation of fluorine-containing groups into biologically relevant molecules has been used as a common strategy for the development of pharmaceutically active molecules. However, the selective incorporation of gem-difluoromethylene groups next to sterically demanding secondary and tertiary alkyl groups remains a challenge. Herein, we report the first cobalt-catalyzed regioselective difluoroalkylation of carboxylic acid salts. The reaction allows for the facile construction of various difluoroalkylated products in good yields tolerating a wide range of functionalities on either reaction partner. The potential of the method is illustrated by the late-stage functionalization of molecules of biological relevance. Mechanistic studies support the in situ formation of a cobalt(I) species and the intermediacy of difluoroalkyl radicals, thus suggesting a Co(I)/Co(II)/Co(III) catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...