Reverse thiophosphorylase activity of a glycoside phosphorylase in the synthesis of an unnatural Manβ1,4GlcNAc library†
Abstract
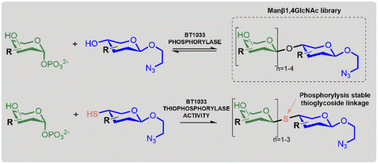
β-Mannosides are ubiquitous in nature, with diverse roles in many biological processes. Notably, Manβ1,4GlcNAc a constituent of the core N-glycan in eukaryotes was recently identified as an immune activator, highlighting its potential for use in immunotherapy. Despite their biological significance, the synthesis of β-mannosidic linkages remains one of the major challenges in glycoscience. Here we present a chemoenzymatic strategy that affords a series of novel unnatural Manβ1,4GlcNAc analogues using the β-1,4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase, BT1033. We show that the presence of fluorine in the GlcNAc acceptor facilitates the formation of longer β-mannan-like glycans. We also pioneer a “reverse thiophosphorylase” enzymatic activity, favouring the synthesis of longer glycans by catalysing the formation of a phosphorolysis-stable thioglycoside linkage, an approach that may be generally applicable to other phosphorylases.
- This article is part of the themed collection: 2023 Chemical Science Covers


 Please wait while we load your content...
Please wait while we load your content...