Mechanistic insights into an NH4OAc-promoted imine dance in Rh-catalysed multicomponent double C–H annulations through an N-retention/exchange dual channel†
Abstract
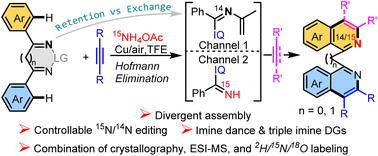
Developing new and understanding multicomponent reactions (MCRs) is an appealing but challenging task. Herein, Rh(III)-catalyzed multicomponent double C–H annulations of cyclic diimines (or diketones and acetone), alkynes, and ammonium acetate to assemble functionalized 1,1′-biisoquinolines and C-bridged 1,1′-bisisoquinolines with controllable 14N/15N editing in one shot has been developed. Through a combination of isotopic-labeling (2H, 18O, and 15N) experiments, crystallography, and time-dependent ESI-MS, the reaction process was studied in detail. Ammonium acetate accounts for two rounds of Hofmann elimination and iminization, thus leading to an unprecedented imine dance, cyclic imine → N-alkenyl imine → NH imine. The N-alkenyl imine can immediately guide a C–H annulation (N-retention channel), and some of it is converted into NH-imine to trigger another annulation (N-exchange channel). The channels and 15N ratios can be regulated by the reaction mode and acidity. Moreover, the resulting 1,1′-biisoquinolines are a privileged ligand scaffold which is exemplified herein by a hydrazine–iodine exchange reaction to form drug-like benzo[c]cinnolines.


 Please wait while we load your content...
Please wait while we load your content...