Dimerization of confined Brønsted acids in enantioselective organocatalytic reactions†
Abstract
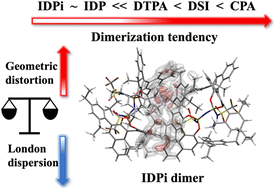
The formation of Brønsted acid aggregates in the course of asymmetric organocatalytic reactions is often overlooked in mechanistic studies, even though it might have a deep impact on the stereo-controlling factors of the transformations. In this work, we shed light on the influence of the catalyst structure and reaction conditions on the spontaneity of the aggregation process for popular chiral organocatalysts derived from phosphoric acids using high-level quantum mechanical calculations. Our study encompasses small and sterically unhindered chiral phosphoric acids as well as large and “confined” imidodiphosphates and imidodiphosphorimidates. These systems have recently proven particularly effective in promoting a large number of highly relevant asymmetric transformations. While cooperative catalytic effects of sterically less hindered chiral phosphoric acid catalysts are well appreciated in literature, it is found that the formation of catalyst dimers in solution is possible for both standard and confined catalysts. The spontaneity of the aggregation process depends on reaction conditions like solvent polarity, polarizability, temperature, the nature of the interaction with the substrate, as well as the catalyst architecture. Finally, it is shown that, at low temperatures (153 K), the aggregation process can profoundly influence the reaction kinetics and selectivity.


 Please wait while we load your content...
Please wait while we load your content...