On-DNA hydroalkylation of N-vinyl heterocycles via photoinduced EDA-complex activation†
Abstract
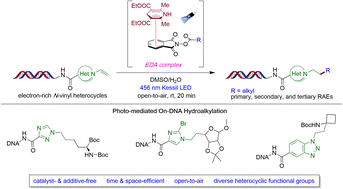
The emergence of DNA-encoded library (DEL) technology has provided a considerable advantage to the pharmaceutical industry in the pursuit of discovering novel therapeutic candidates for their drug development initiatives. This combinatorial technique not only offers a more economical, spatially efficient, and time-saving alternative to the existing ligand discovery methods, but also enables the exploration of additional chemical space by utilizing novel DNA-compatible synthetic transformations to leverage multifunctional building blocks from readily available substructures. In this report, a decarboxylative-based hydroalkylation of DNA-conjugated N-vinyl heterocycles enabled by single-electron transfer (SET) and subsequent hydrogen atom transfer through electron-donor/electron-acceptor (EDA) complex activation is detailed. The simplicity and robustness of this method permits inclusion of a broad array of alkyl radical precursors and DNA-tethered nitrogenous heterocyles to generate medicinally relevant substituted heterocycles with pendant functional groups. Moreover, a successful telescoped route provides the opportunity to access a broad range of intricate structural scaffolds by employing basic carboxylic acid feedstocks.


 Please wait while we load your content...
Please wait while we load your content...