Tellurolate: an effective Te-atom transfer reagent to prepare the triad of group 5 metal bis(tellurides)†
Abstract
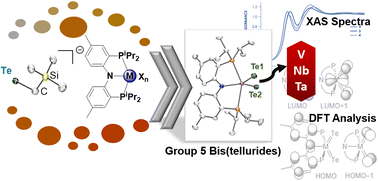
We show in this work how lithium tellurolate Li(X)nTeCH2SiMe3 (X = THF, n = 1, 1; X = 12-crown-4, n = 2, 2), can serve as an effective Te-atom transfer reagent to all group 5 transition metal halide precursors irrespective of the oxidation state. Mononuclear and bis(telluride) complexes, namely (PNP)M(Te)2 (M = V; Nb, 3; Ta, 4; PNP− = N[2-PiPr2-4-methylphenyl]2), are reported herein including structural and spectroscopic data. Whereas the known complex (PNP)V(Te)2 can be readily prepared from the trivalent precursor (PNP)VCl2, two equiv. of tellurolate, and elemental Te partially solubilized with PMe3, complex 3 can also be similarly obtained following the same procedure but with or without a reductant, Na/NaCl. Complex 4 on the other hand is formed from the addition of four equiv. of tellurolate to (PNP)TaF4. Having access to a triad of (PNP)M(Te)2 systems for group 5 metals has allowed us to compare them using a combination of theory and spectroscopy including Te-L1 edge XANES data.


 Please wait while we load your content...
Please wait while we load your content...