Practical synthesis of allylic amines via nickel-catalysed multicomponent coupling of alkenes, aldehydes, and amides†
Abstract
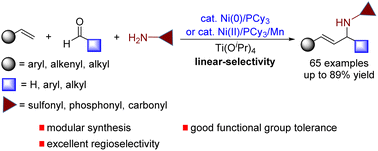
Molecules with an allylic amine motif provide access to important building blocks and versatile applications of biologically relevant chemical space. The need for diverse allylic amines requires the development of increasingly general and modular multicomponent reactions for allylic amine synthesis. Herein, we report an efficient catalytic multicomponent coupling reaction of simple alkenes, aldehydes, and amides by combining nickel catalysis and Lewis acid catalysis, thus providing a practical, environmentally friendly, and modular protocol to build architecturally complex and functionally diverse allylic amines in a single step. The method is remarkably simple, shows broad functional-group tolerance, and facilitates the synthesis of drug-like allylic amines that are not readily accessible by other methods. The utilization of accessible starting materials and inexpensive Ni(II) salt as the alternative precatalyst offers a significant practical advantage. In addition, the practicality of the process was also demonstrated in an efficient, gram-scale preparation of the prostaglandin agonist.


 Please wait while we load your content...
Please wait while we load your content...