Boosting quantum yields and circularly polarized luminescence of penta- and hexahelicenes by doping with two BN-groups†
Abstract
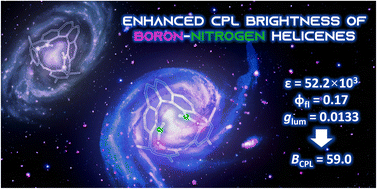
The incorporation of boron–nitrogen (BN) units into polycyclic aromatic hydrocarbons (PAHs) as an isoelectronic replacement of two carbon atoms can significantly improve their optical properties, while the geometries are mostly retained. We report the first non-π-extended penta- and hexahelicenes comprising two aromatic 1,2-azaborinine rings. Comparing them with their all-carbon analogs regarding structural, spectral and (chir)optical properties allowed us to quantify the impact of the heteroatoms. In particular, BN-hexahelicene BN[6] exhibited a crystal structure congruent with its analog CC[6], but displayed a fivefold higher fluorescence quantum yield (φfl = 0.17) and an outstanding luminescence dissymmetry factor (|glum| = 1.33 × 10−2). Such an unusual magnification of both properties at the same time makes BN-helicenes suitable candidates as circularly polarized luminescence emitters for applications in materials science.
- This article is part of the themed collection: 2024 Chemical Science Covers


 Please wait while we load your content...
Please wait while we load your content...