High-throughput determination of enantiopurity in atroposelective synthesis of aryl triazoles†
Abstract
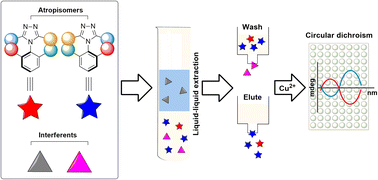
Atropisomeric scaffolds are a common design element found in pharmaceuticals, many deriving from an N–C axis of chirality. The handedness associated with atropisomeric drugs is oftentimes crucial for their efficacy and/or safety. With the increased use of high-throughput screening (HTS) for drug discovery, the need for rapid enantiomeric excess (ee) analysis is needed to keep up with the fast workflow. Here, we describe a circular dichroism (CD) based assay that could be applied to the ee determination of N–C axially chiral triazole derivatives. Analytical samples for CD were prepared from crude mixtures by three sequential steps: liquid–liquid extraction (LLE), a wash-elute, and complexation with Cu(II) triflate. The initial ee measurement of five samples of atropisomer 2 was conducted by the use of a CD spectropolarimeter with a 6-position cell changer, resulting in errors of less than 1% ee. High-throughput ee determination was performed on a CD plate reader using a 96-well plate. A total of 28 atropisomeric samples (14 for 2 and 14 for 3) were screened for ee. The CD readings were completed in 60 seconds with average absolute errors of ±7.2% and 5.7% ee for 2 and 3, respectively.


 Please wait while we load your content...
Please wait while we load your content...