Abstract
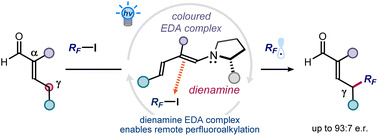
Herein, we report a photochemical organocatalytic method for the asymmetric introduction of perfluoroalkyl fragments (including the valuable trifluoromethyl moiety) at the remote γ-position of α-branched enals. The chemistry exploits the ability of extended enamines (dienamines) to form photoactive electron donor–acceptor (EDA) complexes with perfluoroalkyl iodides, which under blue light irradiation generate radicals through an electron transfer mechanism. The use of a chiral organocatalyst, derived from cis-4-hydroxy-L-proline, secures a consistently high stereocontrol while inferring complete site selectivity for the more distal γ position of the dienamines.


 Please wait while we load your content...
Please wait while we load your content...