Solid-state synthesis of cesium manganese halide nanocrystals in glass with bright and broad red emission for white LEDs†
Abstract
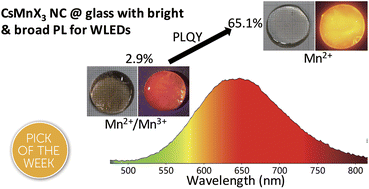
Recently, lead halide perovskite nanocrystals (NCs) have attracted extensive attention due to their unique optical properties. However, the toxicity of lead and the instability to moisture obstruct their further commercial development. Herein, a series of lead-free CsMnX3 (X = Cl, Br, and I) NCs embedded in glasses were synthesized by a high temperature solid-state chemistry method. These NCs embedded in glass can remain stable after soaking in water for 90 days. It is found that increasing the amount of cesium carbonate in the synthesis process can not only prevent the oxidation of Mn2+ to Mn3+ and promote the transparency of glass in the 450–700 nm region, but also significantly increase its photoluminescence quantum yield (PLQY) from 2.9% to 65.1%, which is the highest reported value of the red CsMnX3 NCs so far. Using CsMnBr3 NCs with a red emission peak at 649 nm and full-width-at-half-maximum (FWHM) of 130 nm as the red light source, a white light-emitting diode (LED) device with International Commission on illumination (CIE) coordinates of (0.33, 0.36) and a color rendering index (CRI) of 94 was obtained. These findings, together with future research, are likely to yield stable and bright lead-free NCs for the next generation of solid-state lighting.
- This article is part of the themed collections: 2023 Chemical Science HOT Article Collection, 2023 ChemSci Pick of the Week Collection and 2023 Chemical Science Covers


 Please wait while we load your content...
Please wait while we load your content...