Cooperativity between sodium ions and water molecules facilitates lipid mobility in model cell membranes†
Abstract
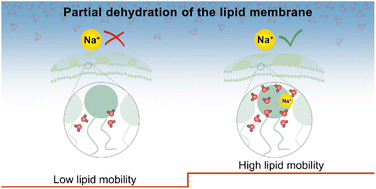
Cellular membranes are surrounded by an aqueous buffer solution containing various ions, which influence the hydration layer of the lipid head groups. At the same time, water molecules hydrating the lipids play a major role in facilitating the organisation and dynamics of membrane lipids. Employing fluorescence microscopy imaging and fluorescence recovery after photobleaching measurements, we demonstrate that the cooperativity between water and sodium (Na+) ions is crucial to maintain lipid mobility upon the removal of the outer hydration layer of the lipid membrane. Under similar hydration conditions, lipid diffusion ceases in the absence of Na+ ions. We find that Na+ ions (and similarly K+ ions) strengthen the water clathrate cage around the lipid phosphocholine headgroup and thus prevent its breaking upon removal of bulk water. Intriguingly, Ca2+ and Mg2+ do not show this effect. In this article, we provide a detailed molecular-level picture of ion specific dependence of lipid mobility and membrane hydration properties.
- This article is part of the themed collections: 2023 Chemical Science HOT Article Collection and #MyFirstChemSci 2023


 Please wait while we load your content...
Please wait while we load your content...