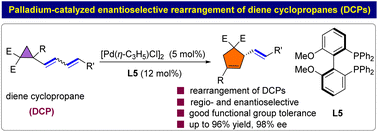
Palladium-catalyzed enantioselective rearrangement of dienyl cyclopropanes†
Abstract
Vinyl cyclopropanes (VCPs) are among the most useful three-carbon building blocks in organic synthesis. They are commonly used as dienophiles in a range of cycloaddition reactions. However, VCP rearrangement has not received much attention since its discovery in 1959. In particular, the enantioselective rearrangement of VCP is synthetically challenging. Herein, we report the first palladium-catalyzed regio- and enantioselective rearrangement of VCPs (dienyl or trienyl cyclopropanes) for the construction of functionalized cyclopentene units in high yields and with excellent enantioselectivities and 100% atom economy. The utility of the current protocol was highlighted by a gram-scale experiment. Moreover, the methodology provides a platform for accessing synthetically useful molecules containing cyclopentanes or cyclopentenes.


 Please wait while we load your content...
Please wait while we load your content...