Metal cations as inorganic structure-directing agents during the synthesis of phillipsite and tobermorite†
Abstract
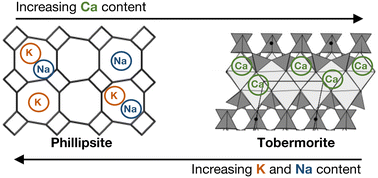
The structure of porous materials in the absence of organic structure-directing agents highlights the adaptable nature of metal cations during hydrothermal synthesis. Here, we perform template-free hydrothermal treatments to synthesize phillipsite and tobermorite, at the same molar precursor ratios, while varying the identity and compositions of the counterbalancing metal cations that act as inorganic structure-directing agents. Phillipsite is crystallized selectively at low total cationic charges (in the recovered solids) in the presence of sodium and potassium at 373 and 393 K. Partial substitution of sodium and potassium with calcium in the synthesis gels results in the co-precipitation of tobermorite phases in proportion to the calcium substitution amount. Exclusive tobermorite precipitation was observed from synthesis growth solutions containing only calcium (373 and 393 K). X-ray diffraction (XRD) patterns, together with nitrogen adsorption isotherms (at 77 K), indicate a monotonic increase in the fraction of tobermorite crystals with increasing calcium content in synthesis gels. Differences in framework topology, dictated by the choice of metal cation, are accentuated by the quantity of metal cation retention within the available and interfacial cavities of phillipsite ((K + Na + Ca)/Al ≤ 1) and tobermorite ((K + Na + Ca)/Al ≥ 1). These results demonstrate the important role of metal cations during crystallization processes and their ability to vary framework topology in porous materials.


 Please wait while we load your content...
Please wait while we load your content...