Kinetics and mechanism of amino acid derived 2-thiohydantoin formation reactions†
Abstract
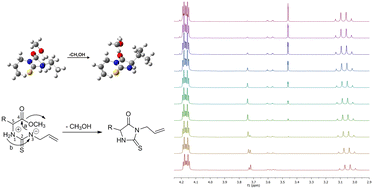
The reaction of allyl isothiocyanate with some common natural protein amino acids (glycine, L-alanine, L-valine, L-leucine and L-phenylalanine) was monitored. 1H NMR spectroscopy is used as a practical tool in searching for the intermediates and determining the kinetic parameters of the reaction. The density functional theory (DFT) method is utilized to better apprehend the mechanisms of thiohydantoin formation and kinetics through which these processes are facilitated, as well as for studying important intermediates and transition states. Based on our findings, a two-step mechanism in which the key reaction step is cyclization via an internal nucleophile in the doubly charged intermediate product is proposed. The theoretical calculations are in good agreement with our experimental observations, and the reactivity of the amino acid esters is compared and discussed.


 Please wait while we load your content...
Please wait while we load your content...