Recent synthetic strategies for the construction of functionalized carbazoles and their heterocyclic motifs enabled by Lewis acids†
Abstract
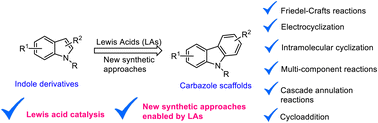
This article demonstrates recent innovative cascade annulation methods for preparing functionalized carbazoles and their related polyaromatic heterocyclic compounds enabled by Lewis acid catalysts. Highly substituted carbazole scaffolds were synthesized via Lewis acid mediated Friedel–Crafts arylation, electrocyclization, intramolecular cyclization, cycloaddition, C–N bond-formations, aromatization and cascade domino reactions, metal-catalyzed, iodine catalyzed reactions and multi-component reactions. This review article mainly focuses on Lewis acid-mediated recent synthetic methods to access a variety of electron-rich and electron-poor functional groups substituted carbazole frameworks in one-pot reactions. Polyaromatic carbazole and their related nitrogen-based heterocyclic compounds were found in several synthetic applications in pharma industries, energy devices, and materials sciences. Moreover, the review paper briefly summarised new synthetic strategies of carbazole preparation approaches will assist academic and pharma industries in identifying innovative protocols for producing poly-functionalized carbazoles and related highly complex heterocyclic compounds and discovering active pharmaceutical drugs or carbazole-based alkaloids and natural products.
- This article is part of the themed collection: 2023 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...