Crafting mono- and novel bis-methylated pyrroloquinoxaline derivatives from a shared precursor and its application in the total synthesis of marinoquinoline A†
Abstract
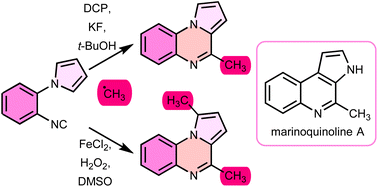
The synthesis of mono- and novel bis-methylated pyrrolo[1,2-a]quinoxalines through the addition of unstable methyl radicals to aryl isocyanides is described contingent upon the reaction conditions employed. The strategy has been effectively employed in the total synthesis of the natural product marinoquinoline A.
- This article is part of the themed collections: Celebrating the scientific accomplishments of RSC Fellows and RSC Advances Outstanding Student Paper Awards 2023


 Please wait while we load your content...
Please wait while we load your content...