Advances in the synthetic strategies of benzoxazoles using 2-aminophenol as a precursor: an up-to-date review
Abstract
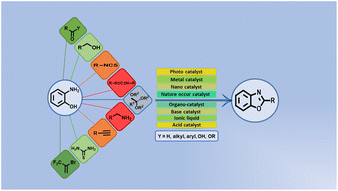
Benzoxazole is a resourceful and important member of the heteroarenes that connects synthetic organic chemistry to medicinal, pharmaceutical, and industrial areas. It is a bicyclic planar molecule and is the most favorable moiety for researchers because it has been extensively used as a starting material for different mechanistic approaches in drug discovery. The motif exhibits a high possibility of broad substrate scope and functionalization to offer several biological activities like anti-microbial, anti-fungal, anti-cancer, anti-oxidant, anti-inflammatory effects, and so on. There has been a large upsurge in the synthesis of benzoxazole via different pathways. The present article presents recent advances in synthetic strategies for benzoxazole derivatives since 2018. A variety of well-organized synthetic methodologies for benzoxazole using 2-aminophenol with aldehydes, ketones, acids, alcohols, isothiocyanates, ortho-esters, and alkynones under different reaction conditions and catalysts, viz. nanocatalysts, metal catalysts, and ionic liquid catalysts, with other miscellaneous techniques has been summarized.
- This article is part of the themed collection: 2023 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...