Anti-Alzheimer activity of new coumarin-based derivatives targeting acetylcholinesterase inhibition†
Abstract
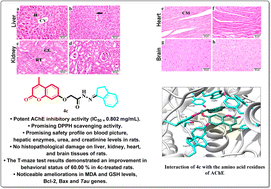
New 2-oxo-chromene-7-oxymethylene acetohydrazide derivatives 4a–d were designed and synthesized with a variety of bioactive chemical fragments. The newly synthesized compounds were evaluated as acetylcholinesterase (AChE) inhibitors and antioxidant agents in comparison to donepezil and ascorbic acid, respectively. Compound 4c exhibited a promising inhibitory impact with an IC50 value of 0.802 μM and DPPH scavenging activity of 57.14 ± 2.77%. Furthermore, biochemical and haematological studies revealed that compound 4c had no effect on the blood profile, hepatic enzyme levels (AST, ALT, and ALP), or total urea in 4c-treated rats compared to the controls. Moreover, the histopathological studies of 4c-treated rats revealed the normal architecture of the hepatic lobules and renal parenchyma, as well as no histopathological damage in the examined hepatic, kidney, heart, and brain tissues. In addition, an in vivo study investigated the amelioration in the cognitive function of AD-rats treated with 4c through the T-maze and beam balance behavioural tests. Also, 4c detectably ameliorated MDA and GSH, reaching 90.64 and 27.17%, respectively, in comparison to the standard drug (90.64% and 35.03% for MDA and GSH, respectively). The molecular docking study exhibited a good fitting of compound 4c in the active site of the AChE enzyme and a promising safety profile. Compound 4c exhibited a promising anti-Alzheimer's disease efficiency compared to the standard drug donepezil.
- This article is part of the themed collection: 2023 RSC Advances Popular Advances Collection


 Please wait while we load your content...
Please wait while we load your content...