Highly stable Fe/CeO2 catalyst for the reverse water gas shift reaction in the presence of H2S†
Abstract
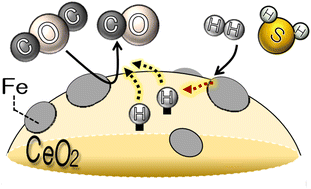
This study focused on evaluating the catalytic properties for the reverse water gas shift reaction (RWGS: CO2 + H2 → CO + H2O ΔH0 = 42.1 kJ mol−1) in the presence of hydrogen sulfide (H2S) over a Fe/CeO2 catalyst, commercial Cu–Zn catalyst for the WGS reaction (MDC-7), and Co–Mo catalyst for hydrocarbon desulfurization. The Fe/CeO2 catalyst exhibited a relatively high catalytic activity to RWGS, compared to the commercial MDC-7 and Co–Mo catalysts. In addition, the Fe/CeO2 catalyst showed stable performance in the RWGS environment that contained high concentrations of H2S. The role of co-feeding H2S was investigated over the Fe/CeO2 catalyst by the temperature programmed reaction (TPR) of CO2 and H2 in the presence of H2S. The result of TPR indicated that the co-feeding H2S might enhance RWGS performance due to H2S acting as the hydrogen source to reduce CO2.


 Please wait while we load your content...
Please wait while we load your content...