1,10-Phenanthroline-based periodic mesoporous organosilica: from its synthesis to its application in the cobalt-catalyzed alkyne hydrosilylation†
Abstract
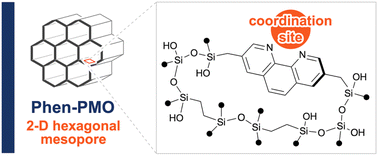
1,10-Phenanthroline (Phen) is a typical ligand for metal complexation and various metal/Phen complexes have been applied as a catalyst in several organic transformations. This study reports the synthesis of a Phen-based periodic mesoporous organosilica (Phen-PMO) with the Phen moieties being directly incorporated into the organosilica framework. The Phen-PMO precursor, 3,8-bis[(triisopropoxysilyl)methyl]-1,10-phenanthroline (1a), was prepared via the Kumada–Tamao–Corriu cross-coupling of 3,8-dibromo-1,10-phenanthroline and [(triisopropoxysilyl)methyl]magnesium chloride. The co-condensation of 1a and 1,2-bis(triethoxysilyl)ethane in the presence of P123 as the template surfactant afforded Phen-PMO 3 with an ordered 2-D hexagonal mesoporous structure as confirmed by nitrogen adsorption/desorption measurements, X-ray diffraction, and transition electron microscopy. Co(OAc)2 was immobilized on Phen-PMO 3, and the obtained complex showed good catalytic activity for the hydrosilylation reaction of phenylacetylene with phenylsilane.


 Please wait while we load your content...
Please wait while we load your content...