How a single mutation alters the protein structure: a simulation investigation on protein tyrosine phosphatase SHP2†
Abstract
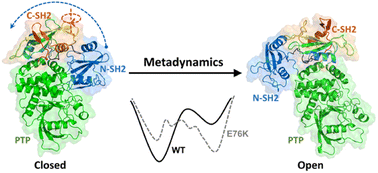
Protein tyrosine phosphatase SHP2 is a key regulator modulating several signaling pathways. The oncogenic mutation E76K in SHP2 releases the enzyme from an autoinhibited, closed conformation into an active, open conformation. Here, we investigated the conformational dynamics of SHP2 and the effect of the E76K mutation on its conformational ensemble via extensive molecular dynamics (MD) and metadynamics (MetaD) simulations. Our simulations provide atomistic details on how the E76K mutated SHP2 prefers the open state and also reveal that the transition between the closed and the open states is highly collective. Several intermediate metastable states during the conformational transition between the closed and the open states were also investigated. Understanding how the single E76K mutation induces the conformational change in SHP2 could facilitate the further design of SHP2 inhibitors.
- This article is part of the themed collection: New Insights into Biomolecular Systems from Large-Scale Simulations


 Please wait while we load your content...
Please wait while we load your content...