Asymmetric synthesis of metallacarboranes using a traceless chiral auxiliary†
Abstract
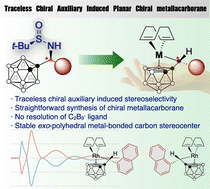
An asymmetric synthesis of chiral metallacarboranes using a traceless chiral N-tertbutylsulfinamide auxiliary is reported. Investigations of the stereoselectivity and mechanism of the unprecedented formation of the metallacarboranes with a planar chiral nido-C2B92− ligand and an exo-polyhedral metal-substituted carbon stereocenter reveal a stereospecific SN2-type reaction with a transition-metal nucleophile and an N-based leaving group. The findings may open a way for the preparation of chiral metallacarboranes and related compounds.


 Please wait while we load your content...
Please wait while we load your content...