Repercussions of multi-electron uptake by a twistacene: a reduction-induced double dehydrogenative annulation†
Abstract
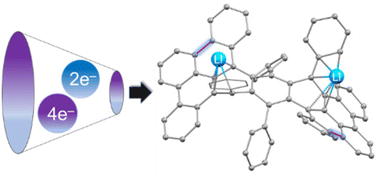
Chemical reduction of highly-twisted 9,10,11,20,21,22-hexaphenyltetrabenzo[a,c,l,n]pentacene (C74H46, 1) was investigated using Li and Cs metals as the reducing agents. The Cs-induced reduction of 1 in the presence of 18-crown-6 ether enabled the isolation of a solvent-separated ion pair (SSIP) with a “naked” monoanion. Upon reduction with Li metal, a double reductive dehydrogenative annulation of 1 was observed to afford a new C74H422− dianion. The latter was shown to undergo a further reduction to C74H424− without additional core transformation. All products were characterized by single-crystal X-ray diffraction and spectroscopic methods. Subsequent in-depth theoretical analysis of one vs. two and four electron uptake by 1 provided insights into how the changes of geometry, aromaticity and charge facilitated the core transformation of twistacene observed upon two-fold reduction. These experimental and theoretical results pave the way to understanding of the reduction-induced core transformations of highly twisted and strained π-systems.


 Please wait while we load your content...
Please wait while we load your content...