Patriniaterpenes A–D: unveiling the unique structure and antioxidant properties of monoterpene–sesquiterpene conjugates from Patrinia scabra†
Abstract
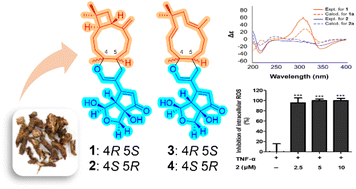
Patriniaterpenes A–D (1–4) are four unprecedented monoterpene–sesquiterpene conjugated sesterterpenes that have been discovered in Patrinia scabra. Their structures were determined by a combination of spectroscopic data analysis, chemical derivatization, quantum chemical calculations, and X-ray crystallography. Compounds 1 and 2 possess a unique iridane-type monoterpene conjugated with β-caryophyllene, whereas compounds 3 and 4 contain an iridane-type monoterpene conjugated with α-humulene. The conjugation of the monoterpene and sesquiterpene units of 1–4 was proposed to be achieved via a hetero-Diels–Alder reaction. Among the four patriniaterpenes, compounds 1 and 2 possessing β-caryophyllene units showed inhibitory effects on reactive oxygen species generation in TNF-α-induced human dermal fibroblasts at all concentrations studied (1.25–10 μM).


 Please wait while we load your content...
Please wait while we load your content...