N-Amino pyridinium salts in organic synthesis
Abstract
C–N bond forming reactions hold immense significance to synthetic organic chemistry. In pursuit of efficient methods for the introduction of nitrogen in organic small molecules, myriad synthetic methods have been developed, and methods based on both nucleophilic and electrophilic aminating reagents have received sustained research effort. In response to continued challenges – the need for substrate prefunctionalization, the requirement for vestigial N-activating groups, and the need to incorporate nitrogen in ever more complex molecular settings – the development of novel aminating reagents remains a central challenge in method development. N-Aminopyridinums and their derivatives have recently emerged as a class of bifunctional aminating reagents, which combine N-centered nucleophilicity with latent electrophilic or radical reactivity by virtue of the reducible N–N bond, with broad synthetic potential. Here, we summarize the synthesis and reactivity of N-aminopyridinium salts relevant to organic synthesis. The preparation and application of these reagents in photocatalyzed and metal-catalyzed transformations are discussed, showcasing the reactivity in the context of bifunctional platform and its potential for innovation in the field.
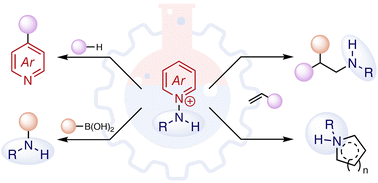
- This article is part of the themed collections: Organic Chemistry Frontiers Emerging Investigator Series 2022–2023 and 2023 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...