Zinc-catalyzed desymmetric hydrosilylation of monosubstituted malonic esters†
Abstract
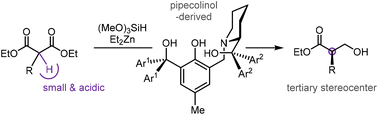
Substituted malonic esters are valuable substrates for desymmetrization to tertiary and quaternary stereocenters, as they can be easily accessed via substitution and the resulting chiral monoesters are versatile building blocks and prevalent motifs in bioactive molecules. Here, building upon a previously reported dinuclear zinc-catalyzed asymmetric hydrosilylation that generated quaternary stereocenters, a pipecolinol-derived tetradentate ligand was devised to extend the desymmetric protocol to monosubstituted malonic esters. This new variation of the desymmetrization has allowed the preparation of structurally diverse tertiary stereocenters in good yields and enantioselectivity. The synthetic utility of these enantioenriched products has also been illustrated in a mild amination procedure to synthesize chiral amino alcohols.
- This article is part of the themed collection: Organic Chemistry Frontiers Emerging Investigator Series 2022–2023


 Please wait while we load your content...
Please wait while we load your content...