Construction of 1,2,4-triazole-fused heterocycles via an amine oxidase-inspired catalyst†
Abstract
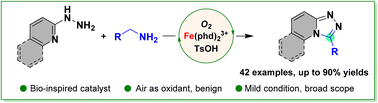
1,2,4-Triazoles are ubiquitous in pharmaceuticals, natural products, coordination chemistry, agrochemicals, and materials science research. Herein we report a practical regioselective synthesis of 1,2,4-triazoles utilizing amine oxidase-inspired catalysis. An o-quino-enzyme mimicking 1,10-phenanthroline-5,6-dione (phd) catalyzed the coupling of two readily available nucleophilic components, a primary amine and hydrazine, in the presence of a Lewis acid (FeCl3) as a co-catalyst using oxygen (1 atm) as the terminal oxidant. The method is atom-economical and environmentally benign, producing water and ammonia as the only by-products. Furthermore, it exhibits a low environmental factor, eco-scale penalty, and process mass intensity. The bio-mimicking protocol affords molecules with varying structural diversity, synthetic bioactivity and drug linkages, and several fascinating physical features. The mechanistic analysis elucidates the executive role of the o-quinone catalyst in the multi-step cascade reaction involving primary amine oxidation and formation of the C–N bond via a sequence of dynamic transamination, electrocyclization, and secondary amine dehydrogenation.


 Please wait while we load your content...
Please wait while we load your content...