Cr-catalyzed chiral allenone synthesis via sequential radical–polar crossover and Oppenauer oxidation†
Abstract
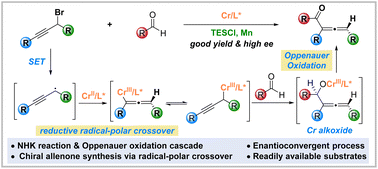
The Nozaki–Hiyama–Kishi (NHK) reaction is a powerful and reliable tool in chiral alcohol synthesis and finds broad application in the total synthesis of biologically important molecules. However, chiral ketone synthesis in NHK reactions remains a prominent challenge. Herein, we describe the chromium-catalyzed enantioconvergent synthesis of chiral 2,3-allenones from readily available aldehydes and racemic propargyl bromides. This method features an unprecedented cascade of Cr-catalyzed asymmetric reductive radical–polar crossover and Oppenauer oxidation, which serves as a new tool for chiral ketone synthesis. Preliminary mechanistic studies provide insights into the reaction process. Kinetic isotope effect studies suggest that the asymmetric addition of an organochromium complex to the aldehyde, instead of the Oppenauer oxidation, might be the rate-determining step.
- This article is part of the themed collections: Organic Chemistry Frontiers Emerging Investigator Series 2022–2023 and 2023 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...